Dilution Questions And Answers . (a) 2.00 l of 18.5 m h 2 so 4,. How much of it do you need to prepare 50. There’s a bottle of 0.750 m nacl on a shelf. I add 560 ml more water to it? 2) if i dilute 250 ml of 0.10 m. Dilution m 1v 1=m 2v 2 1. 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. To dilute a solution means to add more solvent without the addition of more. calculate the number of moles and the mass of the solute in each of the following solutions: dilutions worksheet 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted. a dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without.
from www.chegg.com
2) if i dilute 250 ml of 0.10 m. How much of it do you need to prepare 50. There’s a bottle of 0.750 m nacl on a shelf. a dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without. (a) 2.00 l of 18.5 m h 2 so 4,. 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. dilutions worksheet 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted. To dilute a solution means to add more solvent without the addition of more. Dilution m 1v 1=m 2v 2 1. calculate the number of moles and the mass of the solute in each of the following solutions:
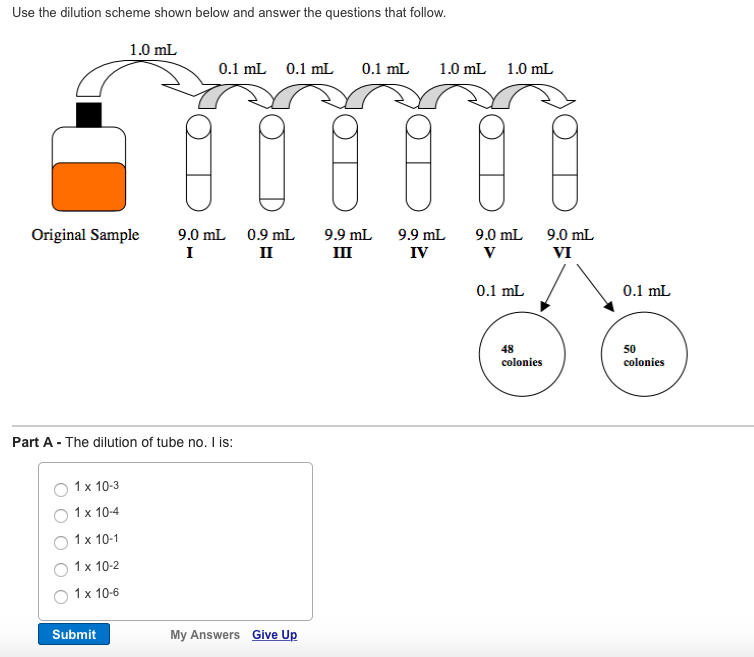
Solved Use the dilution scheme shown below and answer the
Dilution Questions And Answers Dilution m 1v 1=m 2v 2 1. a dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without. Dilution m 1v 1=m 2v 2 1. There’s a bottle of 0.750 m nacl on a shelf. I add 560 ml more water to it? (a) 2.00 l of 18.5 m h 2 so 4,. 2) if i dilute 250 ml of 0.10 m. calculate the number of moles and the mass of the solute in each of the following solutions: dilutions worksheet 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted. 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. To dilute a solution means to add more solvent without the addition of more. How much of it do you need to prepare 50.
From www.chegg.com
Solved For the following serial dilution fill in the blanks, Dilution Questions And Answers How much of it do you need to prepare 50. 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. There’s a bottle of 0.750 m nacl on a shelf. dilutions worksheet 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will. Dilution Questions And Answers.
From www.youtube.com
A Level Chemistry Dilution Calculations Worked Example YouTube Dilution Questions And Answers (a) 2.00 l of 18.5 m h 2 so 4,. Dilution m 1v 1=m 2v 2 1. How much of it do you need to prepare 50. There’s a bottle of 0.750 m nacl on a shelf. 2) if i dilute 250 ml of 0.10 m. a dilution is a process where the concentration of a solution is lowered. Dilution Questions And Answers.
From www.showme.com
ShowMe Dilutions Dilution Questions And Answers calculate the number of moles and the mass of the solute in each of the following solutions: 2) if i dilute 250 ml of 0.10 m. a dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without. (a) 2.00 l of 18.5 m h 2 so 4,. dilutions. Dilution Questions And Answers.
From www.chegg.com
Solved Dilutions 1) In a 1 to 10 dilution, how many parts of Dilution Questions And Answers calculate the number of moles and the mass of the solute in each of the following solutions: To dilute a solution means to add more solvent without the addition of more. 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. I add 560 ml more water to it? dilutions. Dilution Questions And Answers.
From www.chegg.com
Solved 5. 1. There are two ways to prepare dilutions Dilution Questions And Answers 2) if i dilute 250 ml of 0.10 m. There’s a bottle of 0.750 m nacl on a shelf. To dilute a solution means to add more solvent without the addition of more. I add 560 ml more water to it? How much of it do you need to prepare 50. 1) if i have 340 ml of a 0.5. Dilution Questions And Answers.
From worksheetkrause.z19..core.windows.net
Dilution Problems Chemistry Worksheet Dilution Questions And Answers To dilute a solution means to add more solvent without the addition of more. calculate the number of moles and the mass of the solute in each of the following solutions: Dilution m 1v 1=m 2v 2 1. I add 560 ml more water to it? dilutions worksheet 1) if i add 25 ml of water to 125. Dilution Questions And Answers.
From www.studocu.com
Dilutions This is a set of 11 questions on dilutions. Try and use the Dilution Questions And Answers a dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without. 2) if i dilute 250 ml of 0.10 m. Dilution m 1v 1=m 2v 2 1. (a) 2.00 l of 18.5 m h 2 so 4,. calculate the number of moles and the mass of the solute in. Dilution Questions And Answers.
From www.chegg.com
Solved Serial Dilution Questions Fill in the missing numbers Dilution Questions And Answers I add 560 ml more water to it? (a) 2.00 l of 18.5 m h 2 so 4,. dilutions worksheet 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted. To dilute a solution means to add more solvent without the addition of more. Dilution. Dilution Questions And Answers.
From www.docsity.com
Making Dilutions Worksheet Answers Key Docsity Dilution Questions And Answers 2) if i dilute 250 ml of 0.10 m. a dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without. To dilute a solution means to add more solvent without the addition of more. How much of it do you need to prepare 50. 1) if i have 340 ml. Dilution Questions And Answers.
From www.chegg.com
Solved Prelab Questions 1) When making a dilution series, Dilution Questions And Answers I add 560 ml more water to it? calculate the number of moles and the mass of the solute in each of the following solutions: There’s a bottle of 0.750 m nacl on a shelf. Dilution m 1v 1=m 2v 2 1. To dilute a solution means to add more solvent without the addition of more. 1) if i. Dilution Questions And Answers.
From www.chegg.com
Solved Serial dilution is a common technique used in Dilution Questions And Answers I add 560 ml more water to it? How much of it do you need to prepare 50. Dilution m 1v 1=m 2v 2 1. To dilute a solution means to add more solvent without the addition of more. 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. calculate the. Dilution Questions And Answers.
From bryantkruwnavarro.blogspot.com
When Diluting a Solution Which of the Following Changes BryantkruwNavarro Dilution Questions And Answers There’s a bottle of 0.750 m nacl on a shelf. 2) if i dilute 250 ml of 0.10 m. I add 560 ml more water to it? dilutions worksheet 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted. calculate the number of moles. Dilution Questions And Answers.
From printablejacob.z22..core.windows.net
Molarity And Dilution Worksheet Dilution Questions And Answers (a) 2.00 l of 18.5 m h 2 so 4,. There’s a bottle of 0.750 m nacl on a shelf. calculate the number of moles and the mass of the solute in each of the following solutions: How much of it do you need to prepare 50. dilutions worksheet 1) if i add 25 ml of water to. Dilution Questions And Answers.
From www.youtube.com
Dilution Problems Chemistry Tutorial YouTube Dilution Questions And Answers 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. I add 560 ml more water to it? How much of it do you need to prepare 50. 2) if i dilute 250 ml of 0.10 m. There’s a bottle of 0.750 m nacl on a shelf. dilutions worksheet 1) if. Dilution Questions And Answers.
From www.chegg.com
Solved Assignment 1. Dilutions. Question You are given 450 Dilution Questions And Answers 2) if i dilute 250 ml of 0.10 m. How much of it do you need to prepare 50. calculate the number of moles and the mass of the solute in each of the following solutions: (a) 2.00 l of 18.5 m h 2 so 4,. a dilution is a process where the concentration of a solution is. Dilution Questions And Answers.
From www.chegg.com
Solved Use the the scheme shown below and answer the the Dilution Questions And Answers How much of it do you need to prepare 50. (a) 2.00 l of 18.5 m h 2 so 4,. Dilution m 1v 1=m 2v 2 1. a dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without. 1) if i have 340 ml of a 0.5 m nabr solution,. Dilution Questions And Answers.
From www.studocu.com
Complex Dilutions Questions Answers 7PY023 Complex dilutions Dilution Questions And Answers Dilution m 1v 1=m 2v 2 1. There’s a bottle of 0.750 m nacl on a shelf. calculate the number of moles and the mass of the solute in each of the following solutions: 2) if i dilute 250 ml of 0.10 m. (a) 2.00 l of 18.5 m h 2 so 4,. dilutions worksheet 1) if i. Dilution Questions And Answers.
From www.chegg.com
Solved Use the dilution scheme shown below and answer the Dilution Questions And Answers To dilute a solution means to add more solvent without the addition of more. Dilution m 1v 1=m 2v 2 1. (a) 2.00 l of 18.5 m h 2 so 4,. a dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without. 2) if i dilute 250 ml of 0.10. Dilution Questions And Answers.